Authors: Ufuoma Joseph Udi, Mustafasanie M. Yussof, Kabiru Musa Ayagi, Chiara Bedon and Mohd Khairul Kamarudin
Source: Ain Shams Engineering Journal, Volume 14, Issue 5, May 2023, ScienceDirect, Elsevier B.V.
DOI: https://doi.org/10.1016/j.asej.2022.101970
Abstract
Several factors, including incentives associated with aesthetics, transparency, high chemical, and mechanical durability, and its excellent corrosion resistance, have rapidly accelerated the interest and use of glass as windows, façades, or load-bearing elements in structural applications. Nonetheless, the glass is chemically attacked when subjected to certain environmental conditions and its chemistry, structure, as well as its optical and mechanical properties, are altered by the different weathering processes throughout its service life. Several techniques exist for evaluating the performance of weathered glass. These include both natural and artificial ageing techniques. However, little correlation has been shown to exist between natural and artificial ageing, especially the comprehensive comparison between the naturally aged and artificially weathered glazing systems have yet to be examined. In this review paper, the weathering of structural glass systems when exposed to environmental conditions is presented. Emphasis in the literature has been placed chiefly on the different types of glazing in the construction industry and their resistance to three main weathering agents: humidity, temperature, and soiling. Main optical and mechanical tests reported in the literature are summarized, and the properties described in each of them are examined, providing evidence of current challenges, limitations, and insight on future prospects.
1. Introduction
Silicate-based glasses, which are widely used as a construction material and historically the longest-running form of glasses for architectural purposes, have been in existence for over 2000 years. Its growth, which has been particularly notable in the building industry in recent decades, has also been prominent in other sectors such as energy and the automotive and aircraft industries. Judging by the industry market surveys, the global demand for these types of glasses is projected once again to expand at a rate of 6.7 % per annum over the next few years (Fig. 1) [1].
![Fig. 1. Global flat glass demand trend [1].](/sites/default/files/inline-images/Fig1_312.jpg)
Between 2012 and 2019, the global construction sector is estimated to have utilized 75 million metric tons of flat glass per year. The construction sector consumed a large share (70 %) of the entire flat glass produced. Considering the usage of flat glass has expanded in recent years, the proportion employed in the construction sector is expected to rise. In addition, the building sector of the Asia-Pacific region in particular—which has seen rapid expansion in recent years due largely to a combination of fast-growing economies, increased infrastructure expenditures, rapid urbanization, and the sustained rise in popularity of smart city schemes—is expected to be the driving force of flat glass demand [2].
The attraction of this material can be comfortably justified considering its remarkable advantages such as desirable aesthetics, high transparency, good chemical and mechanical durability, as well as its excellent corrosion resistance, and considerably small energy consumption for its manufacturing [3], [4], [5], [6], [7], [8]. Besides its overall appeal, the use of glass has also been driven largely by comfort considerations e.g., providing thermal comfort for users, creating a visual and psychological connection between the interior and exterior environment, and also reducing energy consumption for artificial lightning [9], [10], [11], [12].
These glasses, however, are enriched with alkaline and alkaline earth oxides, employed as fluxes in lowering the glass forming mixture’s viscosity and melting temperature. As a result, silicate glasses could experience significant optical changes involving the chemical reactions with atmospheric agents that may impair their long-term durability. This alteration process is mainly dependent on the chemical composition of the glass, including environmental factors like relative humidity, temperature, airborne particulate matter, and acidifying gases.
Typical characteristics of glass alteration include network dissolution and the formation of gel layers owing to the presence of atmospheric water, as well as crystalline product formation, necessitated by airborne pollutants dissolving on the glazing surfaces [13], [14], [15]. Moreover, the brittleness of the glass material renders it vulnerable to micro-cracks and surface flaws [16], which accumulate throughout its service life as a result of abrasion, impact, or contact. This consequently leads to a reduction in the glass extrinsic strength, thus degrading its mechanical performance over time. These deterioration phenomena are generally known as weathering.
Weathering of silicate glasses under seemingly innocuous environments has been extensively studied for several decades. Studies pertaining to glass surface alteration due to atmospheric agents were initiated in the 1950 s. Most of these studies specifically dealt with the alteration mechanisms on artificially aged glass—a technique that is employed to expedite the natural weathering processes—with relatively far fewer on naturally weathered glass. Nonetheless, the damages which are induced by the artificial ageing process bear some specific concerns, as will be highlighted in this paper. The significance of glass weathering stems from the necessity of researchers to better understand the durability of aged glazing and to have knowledge of its deterioration mechanism under environmental conditions to ensure their continuous and safe usage in load-bearing applications.
This paper presents a review of existing experimental studies related to the weathering of soda-lime-silicate glass, focusing particularly on the natural and artificial ageing mechanisms of this glass type. In section 2, the physical and chemical properties of standard construction glasses are reported, providing support with multiple referenced literature findings. Due attention is given, particularly to material characteristics constituting important parameters in silicate glass, and an overview of the glass weathering process as well as the factors influencing this phenomenon. 3 Existing experimental research on weathered structural glass, 4 Retrofitting and strength enhancement of weathered structural glass discuss existing experimental research on the effect of environmental factors on the mechanical and optical properties of silicate glass, with careful consideration on natural and artificial ageing of different construction glass systems and weathered glass retrofitted via protective films. Section 5 finally presents the remaining issues that should be resolved so as to develop improved solutions for environmentally impacted glazing in structural applications.
2. General principles and concepts
2.1. Chemical durability of glass; strength and properties
A large percentage of float glass in existing or novel structures comprises a significant proportion of multiple metallic oxides, notably Na2O and CaO, in addition to the primary element, silica (usually referred to as sand). Hence, due to the raw materials, it is referred to ‘as soda-lime glass’ or ‘soda-lime-silicate (SLS) glass’ [17]. Other variants of silica glass with unique characteristics include borosilicate glass, aluminosilicate glass, and lead-oxide glass (crystal glass). Buildings utilizing such glasses are indeed limited compared to the large number of soda lime silicate glass used for façades, window glass, load-carrying elements, etc., which is the focus of this present study.
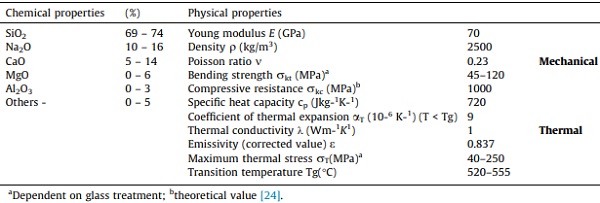
In addition to their transparency, a beneficial characteristic of SLS glasses that has rendered them valuable throughout history for several applications is their chemical durability, which is exceptionally high for pure silica glass. The chemical durability of SLS glass alludes to the fact that the rate of surface alteration is highly dependent on the chemical composition of the glass, i.e., it is a feature of the total glass composition. For economic and practical purposes, the main chemical parameters in SLS glass and glasses in the glass industry are the contents in CaO and the respective proportions in silica and sodium oxides. For example, since sodium ions are so soluble in aqueous solutions, the addition of oxides like CaO enhances the chemical durability of the glasses due to the increase in relative stability against dissolution, i.e., fewer soluble dissolution products is formed, which is dependent on the pH level [18], [19]. The primary physical and chemical properties of SLS glass at room temperature are reported in Table 1. In general, at the initial stage of glass surface alteration, the chemical composition is believed to possess a vital influence in defining the rate-controlling process after which a hydrated layer forms. The chemical compositions of the glass also have a significant impact on the vastly differing kinetics of hydration in glasses.
Table 1. Physical and chemical properties of soda-lime-silica glass [23].
In terms of mechanical properties, the isotropic, nearly perfectly elastic property identified in glass coupled with its inability to yield plastically reflects this material’s brittle behavior. Glass is generally tough in compression and weaker in tension. Therefore, failure will most likely occur in tension by brittle fracture without warning because the material cannot redistribute local stress concentrations. Theoretically, the strength of glass is incredibly high due to its molecular forces, but for structural applications, this property is of no practical importance. The important engineering property: the actual tensile strength, however, is much lower [20], [21], [22]. The reason for the variation in the practical and theoretical strength of glass is due to the fundamental differences between linear-elastic brittle materials. Moreso, the limit state of brittle materials such as glass is defect-sensitive and several surface flaws exist in their initial condition, including technological and operational mishaps that unpredictably influences the glass strength. These technological defects and operational damages could be in the form of the residual stress of the glass, action history (duration and intensity), size of the glass, condition of the surface, and environmental conditions [22]. The strength values are therefore statistically inhomogeneous.
2.2. Glass in construction
As is well attested, a variety of construction glass systems are readily accessible in the market, and annealed (AN) float glass represents the primary base material. As stated previously, the glass alteration strongly depends on the surface chemical composition, which could differ from the bulk composition as a result of the processing parameters [25]. For instance, the bottom side of AN glass, which is tin-enriched, is more chemically durable than the airside [26]. Likewise, the inclusion of SO2 inside the annealing lehr induces surface dealkalization [27], which further enhances their durability. In general, SLS glass exhibits strong chemical durability against most acids. However, mechanical strength remains the notable concern of AN glass for its usage in various applications.
Annealed Glass (AN) has modest bending strength (up to 45 MPa its characteristic nominal value) and is also relatively economic [28]. For the purpose of simplicity, Table 1 also shows nominal values for glass mechanical properties. Chemical or thermal procedures can then be employed to improve the strength of AN glass, resulting in chemically or fully tempered glasses, i.e., toughened glass (tensile resistance up to 120 MPa) [29]. These strengthened glasses also have added advantages such as improved tensile strength and other advantageous characteristics, particularly in lieu of size and shape of fragments in the event of an inadvertent collapse.
Experimental techniques proposed in leading design codes such as the ISO 1288–2 [30], to assess glazing strength include four-point bending test and coaxial double ring (CDR) test. Nonetheless, the strength result calculated via experimentation for a set of test specimens might not correlate to any acceptable probability distribution (Weibull probability functions are generally recommended in design codes) [31].
2.2.1. Laminated safety glass
Laminated glass represents an arrangement of usually-two plies of glass glued together by foils comprising of certain types of bonding films commonly known as interlayer. As a general rule, the resistant cross-section of the laminate is intended to conform as a composite in reaction to extrinsic load. Thus, better mechanical performance in both elastic and post-cracking phases than a single glass pane. A tacit benefit of laminated glass in structural applications from a mechanical perspective is that two or more glass sheets could be glued together [32], [33], [34], [35] (Fig. 2). Therefore, the necessary degree of strength and stiffness could be achieved by utilizing standard glass thickness accessible in the market. Moreover, due to the advent of bonding films, laminated glass has been the conventional safety option in buildings for decades because the interlayer can strap glass fragments together in the event of a collapse.
![Fig. 2. (a) Example of fractured glass, (b) Laminated glass with interlayer, and (c) load–temperature behavior of laminated glass with three different interlayers [35].](/sites/default/files/inline-images/Fig2_320.jpg)
Bonding films usually comprise Polyvinyl butyral (PVB) films. Over the last several decades, PVB is arguably the most prevalent interlayer used in the construction glass industry. Following its introduction in the 1930 s, innovative attempts have centered on measures to make the interlayer itself less expensive to manufacture, less vulnerable to irregularities during lamination, easier to handle, or to improve some of its characteristics [36]. Many optimized PVB products with unique features such as structural function, solar reflection, acoustic insulation, tighter bond, and greater adhesion to glass, as well as security and decorative functions, have appeared as a result of PVB maturity as a laminated glass interlayer. These advancements have enabled it to be used in automotive windshields, PV solar cells, structural glass components, and security glass, in addition to its application in laminated doors and windows [36].
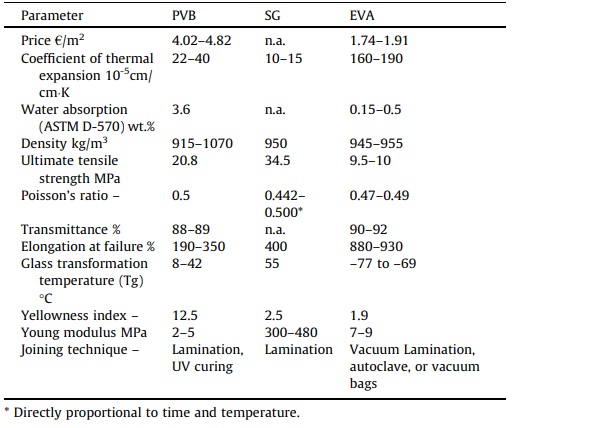
A number of other interlayers could also be employed besides PVB, such as SentryGlas (R), Ethylene-vinyl acetate (EVA), and Polyethylene which were incorporated into the laminated glass sector to fulfill the high-performance resistance requirement for hurricane glazing [37]. SG is an interlayer form of ionic resin or Ionoplast which provides high stiffness and transparency over a broad temperature range (elastic modulus of about 100 MPa for temperatures up to 50 °C). EVA is a sturdy foil made using a plasticized PVB. This material provides excellent flexibility, elasticity, toughness, stress-crack resistance, and clarity as an interlayer material. EVA also possesses specific distinctive characteristics such as high optical transmission, excellent electrical resistivity, resilience to weather conditions, as well as low fusion and polymerization temperature.
However, as highlighted by several scholars [35], [38], [39], [40], [41], [42] and as shown in Fig. 2, a typical feature of these elastomeric materials is that these films are usually governed by their viscous nature in addition to their distinct constitutive principles; thus, are typically prone to chemical modifications relative to the ambient conditions they are exposed to over time. Hence, the mechanical and thermos-viscoelastic properties of the polymeric materials, as well as their adhesion with glass or other substrates such as steel, may be affected on exposure to other environmental stresses like UV radiation, thermal cycles, or humidity conditions [43], [44], [45]. The coupling capability between the different components may also be altered by additional factors such as failure of the bonding layer, material degradation, or delamination which is most prevalent during warm moist weather [46].
In addition, the chemical structure of laminated glass interlayers is the same for all manufacturers. Nonetheless, the unique attributes of each interlayer sheet are dependent on the interlayer type, manufacturer, and composition of the interlayer sheet (Table 2) [42]. Thus, the interlayer properties in addition to its durability and weathering resistance, have a significant impact on the optimal structural integrity of a given laminate component.
Table 2. Main properties of laminated glass interlayers (PVB, SG, and EVA) [42].
2.3. Factors to glass deterioration/failure—Environmental action
Glass deterioration which may lead to failure in buildings jeopardizes the health and safety of the building and its users. Indeed, such an occurrence induces uneasiness, especially when the circumstances surrounding the event are not instantly evident, or in cases where the breakage appears unwarranted relative to the deed which led to the failure. Glass inability to prevent crack propagation or disperse peak stresses render this construction material vulnerable to a wide range of failure factors than those recognized in design codes. These reasons for failure include manufacturing or post-processing issues, surface quality, weathering, and accidental or purposeful impact [47], [48], [49]. Some of these factors which are pertinent to this study are briefly described in the following sub-sections. Table 3 reports a summary of glass failure factors.
Table 3. Factors to glass failure [49].
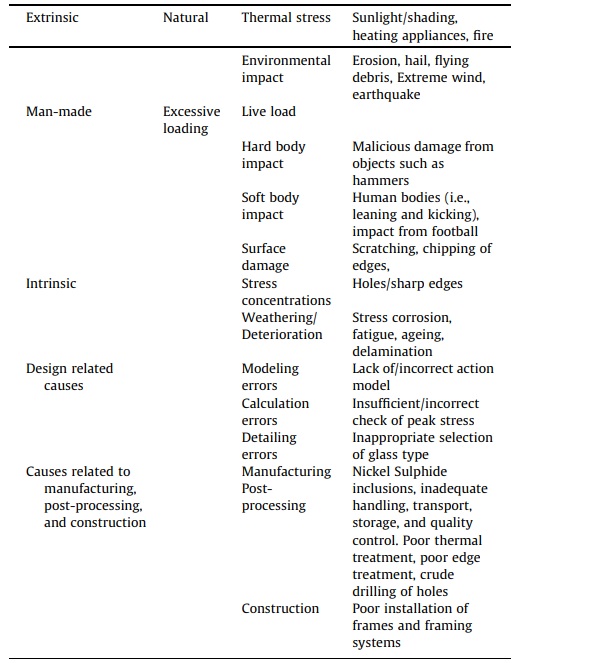
2.3.1. Stress corrosion
Besides its brittle nature and susceptibility to surface flaws and cracks, SLS glass degradation is also influenced by stress corrosion. This deterioration mechanism is attributed to the subcritical propagation of micro-cracks and surface flaws, which results in a decrease in glass extrinsic strength, thus, a loss in its mechanical performance over time. One of the earliest studies by Griffith [50], attributed the cause of this low extrinsic strength to the inevitable occurrence of minuscule defects on the glazing surface—with random orientations and at random locations—introduced at the manufacturing, handling, or installation stages which then accumulates when exposed to environmental conditions throughout its service life. These flaws are commonly described as minuscule or sub-micrometric fractures (acting as stress concentrators) that evade detection under visual examination. Worse than this, these stress concentrators are culpable in a localized amplification of the environment’s corrosive effect on the glazing. Even at an average global stress level, these micro-cracks will typically propagate over time, eventually leading to a prolonged deterioration, particularly under humid conditions [51], [52], [53].
This phenomenon—named static fatigue—is usually more or less gradual at ambient conditions in the unstressed material. However, these flaws could be massively accelerated in an overly strained location at the crack tip, contingent, particularly on the environmental conditions and its confinement. The detailed nature of the stress corrosion phenomenon—which is out of the scope of this present study—has been extensively assessed by Ciccotti [54]. By taking into account the experimental data available in the literature after the 1960 s, the study gave evidence of damage mechanisms in SLS glasses, along with a critical analysis of several processes potentially contributing at various phases in the stress corrosion process.
2.3.2. Erosion and scratching
The mechanical performance of modern construction glazing systems may be evaluated on the basis of their scratching resistance and erosion resistance. Brittle materials such as glass are prone to scratches when materials with superior hardness are pressed onto the glass and scrapped along its surface [55], [56], [57], [58]. Poor handling of the glass during the transportation and installation phase, cleaning, or during in-service conditions can all produce scratches on the glass surface. Indenters or commercially accessible scratching instruments that can support geometrically varied indenter tips are commonly used to assess the scratching resistance of glass elements. Contingent on the extent of the induced damage, scratches can be characterized under the following regimes (i) micro ductile regime associated with a permanent deformation induced on the surface of glass and eventual formation of subsurface lateral cracks, (ii) micro-cracking; median cracks develop while the extended lateral cracks intersect with the glass surface (iii) lastly, the so-called micro-abrasive regime is characterized by the deposition of chips or debris, in addition to the formation of small lateral cracks along its surface. The glass scratch resistance and associated regimes are strongly dependent on the glass chemical composition, environmental conditions, scratching speed, curing regime, as well as the geometry of the indenter, and level of the normal load [58].
Glass erosion typically occurs due to exposure to flying debris and projectiles constantly striking its surface, leading to material removal. Such threats are increased in extreme wind conditions and in areas where windstorms are a frequent occurrence. The erosion resistance can be evaluated either with the sandblasting technique [59], [60], [61], by propelling sand unto the surface of glass using compressed air, or the sand trickling technique [62], [63], in which sand is made to slide gently onto the glazing surface from a predetermined distance. The erosion resistance or damage to the glass depends on the impact velocity, impact angle, the mass of the abrasion agent, size and shape of particles, and abrasion duration [64].
2.4. Weathering of structural glass systems–Overview
Whilst a majority of this section relates to several forms of silicate-based glasses, emphasis is placed on SLS glass employed for engineering and architectural purposes. Unlike many other building materials, SLS glass is very resistant to corrosion, and in a sense, it can be thought of as ‘corrosion proof.’ Nonetheless, in certain environmental conditions, the glass is ‘chemically attacked,' leading to an alteration of its optical and mechanical properties by different weathering processes. The rate and extent to which the glass weathers are highly influenced by the chemical composition of the glass, its manufacturing processes, and the nature of the environment. The main environmental parameters examined in this study are relative humidity (RH), temperature, and airborne pollutants.
2.4.1. Atmospheric water
Weathering of glass by atmospheric water is a major concern that has garnered a lot of interest over the last several decades. Experimental results have shown that water is the primary weathering agent liable for two pivotal phenomena: leaching and corrosion, known as phase 1 and phase 2, respectively. This mechanism involves the network modifier ions (e.g., K+, Na+) present in the glass, as well as some hydrogen-containing ions like H3O+, H+, molecular water or even larger aggregates of the attacking medium. This first mechanism, which takes place up to a pH of approximately 9 in predominantly acidic and neutral environments could also be referred to as the ‘ion-exchange mechanism’ (equation (1)) [65], [66].
![]()
The above reaction selectively leaches glass constituents: the alkali ions on the glass surface becomes diminished, with no dissolution of the silicate network (i.e., it remains intact). The percentage ratio of the network modifiers and silica are substantially smaller than the mass ratio of the glazing, indicating a selective (preferential) leaching of the modifiers. The formed, altered glass (see Fig. 3) is sometimes called a ‘leached layer,’ ‘hydrated glass,’ or ‘gel layer’ and its thickness depends on several internal and external factors. This includes the glass chemical composition, initial surface morphology, its thermal history, the pH of the attacking solution, the concentration nature, the volumetric ratio of liquid solution exposed to the glazing surface area, as well as several environmental parameters like temperature including the exposure duration [14], [67], [68], [69], [70].
![Fig. 3. SEM/EDX photographs of glass surfaces after 12 months weathering in outdoor environment (a–b) weathering products overlaying major portions of the glazing surfaces; (c–d) formation of single crystals consisting of syngenetic products [70].](/sites/default/files/inline-images/Fig3_303.jpg)
At a subsequent phase, the volume of the leached glass layers progresses linearly with time (stage 2) [71]. The transition between the first and second phases occurs in a small timeframe and depends on the chemical composition of the glass and the degree of the reacting temperature. The ion diffusion process is inhibited by an increment in the thickness of forming constituent layer and possible transformation of its structure. Concurrently, the ingestion of hydrogen molecules dissolving on the glazing surface as hydronium ion (H3O+) that is confined to the non-bridging oxygen locations as Si-OH groups (see Equation (2)), increases the pH at the solution/glass interface or possibly throughout the medium [72]. In contrast to the first mechanism, this reaction is accompanied by a congruent dissolution of the glass.
![]()
The kinetic reactions and corrosion processes described therein have been documented in the literature for different glazing materials, utilizing either large glass samples—subjected to different times in a climatic chamber with controlled humidity and temperature—or glass fragments of specific grain size (dipped into the corrosive solution under well-defined laboratory condition). The pH of the final solution and the weight change per unit surface area is traditionally utilized to derive specifics of the weathering mechanisms. Also, in some instances, alkaline ions concentration profile is determined by etching the corrosive glass layer after layer. Atomic absorption spectrophotometry, emission spectroscopy, or flame photometry were employed at the early ages to analyze the leached glass constituents for which appropriate operating standards are described in the literature [73], [74].
However, in recent decades, the depth-profiling and surface analytical techniques, which even allowed in special situations, non-destructive analysis of the chemical or morphological alteration of the glazing surface throughout the leaching phases, has garnered widespread recognition. Some of these techniques include secondary ion mass spectrometry (SIMS) [27], [75], [76], atomic force microscopy (AFM) [27], [77], [78], [79], [80], scanning electron microscopy in conjunction with the energy or wavelength dispersive X-ray spectrometry (SEM/EDX or SEM/WDX) [81], gamma-ray spectrometry or proton-induced X-ray (PIGE or PIXE) [82], as well as nuclear reaction analysis (NRA) or Rutherford backscattering spectrometry (RBS) [77], [83], [84]. In a few cases also X-ray photoelectron spectroscopy (XPS) and elastic recoil detection analysis (ERDA) was utilized for the characterization of the altered glazing surfaces [77], [85], [86], [87]. Finally, the non-destructive spectroscopic ellipsometry (SE) [88], [89] and Auger electron spectroscopy (AES) [90] has received considerable interest in describing glazing surface morphology, including the early phase of the corrosion process, particularly glass weathering.
2.4.2. Atmospheric pollutants
A vast quantity of pollutants (gas and particulate matter) is circulated in the air and can interact on the glazing surface irrespective of their chemical composition and durability. The gravitational settlement of airborne particulates on glass surfaces also impinges on its physicochemical properties. This results in the deterioration of the glass optical properties such as loss of transparency, surface roughness, and increase in wetting time due to condensation of atmospheric water around the particles, thus, exacerbating the leaching process. This type of degradation is commonly known as glass soiling [90], [91], [92]. This phenomenon is generally quantified using the loss of light transmittance [93], [94], [95], reduction in surface gloss [96], [97], [98], or the haze [98], [99], [100], [101]. Besides the contexts of aesthetics and economics (maintenance cost), glass soiling also signifies the toxicity of the natural environment.
Experimental studies detailing the rate and processes of glass soiling are abundant in the literature. In most of these investigations, attempts have been made in describing the nature of particulates deposited on glazing surfaces by subjecting the material, in most cases to chemical analyses and in particular by electron microscopy [91], [98], and UV VIS spectrometer [99], [103]. These experimental investigations demonstrated that the pollutants are composed primarily of gaseous species like NOx, SO2, VOCs, CO2, and secondary pollutants like H2SO₄, HNO3, O3, H2CO3 which are associated with the acidic attack of the substrate [104], [105]. Airborne particulate matter (PM) linked to elemental carbon soot (primarily combustion and automobile exhaust) can conform to the glass surface, thus, altering its physical, chemical, and optical properties. This may ultimately reduce the useful life of the glass material [103], [106], [107], [108]. It has also been established that the soiling rate—defined as the volume of deposits (TP/S) per unit surface area (μg cm−2) of the glass—is dependent on the deposition along with the chemistry and nature of the particulates, as well as the orientation of the glass surface [109].
As shown in Fig. 4, Fig. 5, Fig. 6, all of these variables consolidate in producing a highly site-specific array of deposition and deterioration, i.e., soiling intensity is linked to the nature of pollutants present in the environment (rural, urban, and industrial) [110], [102]. However, the aforementioned studies could only outline the soiling cycle within the time and location where the investigations transpired. Consequently, in-depth knowledge is needed if these findings are to be adapted to different times and locations. This is especially vital if the impact of the projected scenarios and trends of pollutant concentrations and emission are to be assessed with respect to soiling consequences. For this reason, various empirical models have been recommended in the literature to assess the soiling evolution of glazing surfaces with respect to time. This include the square root method [111], [112], different exponential forms [96], [97], or a sigmoid curve, also known as ‘Hill equation’ [113].
![Fig. 4. Atmospheric depositions at rural, urban, and industrial locations (1985–2015) (a) SO2 concentration, (b) NO2 concentration, and (c) O3 concentration [102].](/sites/default/files/inline-images/Fig4_293.jpg)
![Fig. 5. Weathered glass samples as seen under optical microscopy (a) – (b) AN, (c) FT, and (d) Chemically toughened glass [133].](/sites/default/files/inline-images/Fig5_286.jpg)
![Fig. 6. Fig. 8. SEM images of weathered products formed on sheltered glass after six months of exposure (a–b) Na-rich sulphates/carbonates; (c–d) Ca-rich root sulphates [81].](/sites/default/files/inline-images/Fig6_260.jpg)
However, the dose–response function (DRF) is the prominent technique adapted in recent years to evaluate the impact of soiling on glazing surfaces. This statistical method relates a dose (i.e., environmental factors) towards the material’s response, particularly changes to reflectance or haze [114]. A general equation takes the form:
![]()
Where A denotes the amplitude, a function of the meteorological or environmental doses at a particular location and g(t) denotes soiling temporary trends.
The primary aim of the DRF is to estimate soiling evolution over time and also to develop maps displaying geographic variances, as well as to aid in maintaining air quality [115], [116]. In this regard, it imposes some constraints; the environmental factors (RH and temperature) have to be efficiently monitored and their response has to be precisely measured.
2.4.3. Temperature fluctuations
The major concern when architectural glass is subjected to high solar irradiation is the development of thermal fractures—spurred by transient thermal stress due to sudden temperature change—and their resistance to it. In certain climatic regions across the world, air temperatures could rise to 50 °C, and the surface temperature of the glass could surpass this value. This has numerous implications on the material properties of the glass. When exposed directly to sunlight, there is a risk of thermal crack occurring on the glass if the generated stresses surpasses the glazing strength. Thus, the serviceability of the material is reduced over time.
Glass behaves as a linear elastic material within the specified design load as long as the glass transition temperature Tg—given in Table 1—is not exceeded. As a result, considering a set of ordinary loads to validate, understanding the material’s resistance values and elastic properties for SLS glass affords analytical analysis to be conducted using fracture mechanical approaches [117], [118]. However, crack occurrence and propagation due to possible thermal stress could occur prematurely, thus, prompting multidisciplinary approaches for such construction material. Thermal cracks are infact, usually anticipated to develop at temperature gradients of 40 K for AN glass and 200 – 250 K for FT glass [119].
Moreover, formation of crystalline products on the surface of glass could be influenced by high surface temperatures and temperature fluctuations due to increased solar radiation, which could ultimately accelerate the glass corrosion process (diffusion and ion exchange) as well as the forming leached layer [120], [121].
3. Existing experimental research on weathered structural glass
The first consequential studies on the impact of environmental stresses on the properties of structural glass systems came to light towards the end of the 1950 s because of the enormous usage of glass material as window panes. However, many of these research efforts focused on the investigation of thermal stress breakage–being indicative of the leading cause of fracture in window glass [119], [122].
The causes and processes of glass weathering have been extensively studied for several decades. Weathering can occur as a result of either mechanical or chemical action on a glass plate. Mechanical action can be caused as a result of wind debris or abrasion. In contrast, chemical action is due chiefly to the effects of environmental parameters such as temperature, atmospheric water vapor (relative humidity), or airborne pollutants on the plate surface. In either case, the introduction of additional flaws—to the already existing flaws (section 2.3.1)—causes a degradation in the glass properties. The ensuing sections of this paper examines the findings gained from available experimental literature on the distinct characteristics and mechanism of glass alteration when exposed to severe environmental conditions.
3.1. Environmental impacts on glass optical properties
A comprehensive literature review on the deterioration of structural glass systems exposed to environmental conditions has neither been attempted nor possible, and in this present study, particular emphasis will be placed on major experimental techniques and results. The glass literature is laden with experimental support for the ion-exchange/glass-dissolution mechanism described in the previous sections and comparable findings have been obtained over a wide range of reaction conditions and glass composition.
3.1.1. Effect of relative humidity
The majority of the early RH investigations in the context of SLS glass degradation behavior were focused on characterizing and analyzing the mechanism of glazing surface degradation and detailing the second phase decomposition. Also, the influence of the glass chemical composition, humidity, and temperature on the kinetics of leaching and layer formation as well as corrosion-induced structural changes in window glass, were also studied.
For example, Stockdale and Tooley [123] studied the progressive deterioration of glass surfaces after exposure to high humidity conditions with the aid of photomicrographs. Glasses of varying chemical compositions were taken into account—at relative humidity ranging from 55 to 95 % at 90 °C for a total of 1296 h—giving evidence of glass surface alteration due to humidity in a time-dependent manner, as well as furnishing some basis for characterizing the course of leaching. Later on, Simpson [124] studied the durability of various glasses containing different chemical compositions by evaluating the degree of surface deterioration due to moisture with the aid of an electron microscope after subjecting specimens to controlled cyclic humidity tests. In all conditions, the author noted that glass showed surface defects that were dependent on its chemical composition and previous surface treatment. Microscopic comparison of the surfaces of new and weathered glass which was developed through acid etching techniques was also studied by Levengood [125]. The difference between this study and prior research efforts was the weathering of SLS glass by dipping in aqueous solutions. Test results gave evidence—and in accordance with earlier research efforts—of large differences in surface condition appearances between new and weathered glass specimens. The general trend observed and from photographs obtained from these studies showed significant differences in surface conditions exist. This set of works successfully characterizes the dependence of glass leaching on RH and the temperature of the surrounding environment.
The surface characterization technique employed in previous studies was used with good effect by Shelby and Vitko [126] to study the weathering action on the two sides of low-ion float glass. In their study, the authors used various surface characterization methods such as SEM, optical spectroscopy, dye penetration testing, resonant nuclear reaction profiling, and surface profile measurements. 3 mm thick glass plates were taken into account and artificially weathered by exposing the specimens to 98–100 % RH under a well-defined environmental chamber at 50–70 °C for a duration of four weeks. In general, their findings which were specific to each system studied, and by nature very complex at the time, gave evidence—and in accordance with other literature findings [77], [127], [128], [129]—of the airside being more susceptible to corrosion, porosity, and water species penetration than the tin side. The disparity between both studies was ascribed to the improved corrosion resistance of the tin side of float glass. Their findings also indicated the complexities of the underlying mechanisms, implying that the broad generalizations regarding the effect of RH on surface deterioration cannot be transposed to other parameters with success.
Besides transportation and storage, particles also play an important role in outdoor weathering where glass surfaces experience particle exposition as well. Recent studies have shown that particles massively enhance the crystallization process in acidic environment [130], which could lead to drastic network dissolution during ongoing weathering. As such, knowledge on basic interactions of particles with glass is necessary to decide on needed cleaning cycles to avoid a change in surface properties or even a loss in light transmission and thus, a long term degradation of glass. In this regard, Reiβ et al. [79] described an articulated experimental campaign on the chemical changes of SLS glass surfaces caused by Moroccan Sahara sand containing quartz (69.3 wt-%), albite (27.5 wt-%) and magnetite (3.1 wt-%). The authors used various surface characterization methods such as AFM, XPS, XRD, and EDX to investigate the chemical changes of the glasses. Results from the study—after 8 days of weathering in a climatic chamber—gave evidence of sand drastically enhancing the leaching of network modifiers, especially of Na, despite a non-existing acidic environment. Apart from faster leaching dynamics, the sand massively influences crystallization processes and the chemistry of glass surfaces: The formation of carbonates is suppressed and the formation of zeolithes, which could lead to drastic network dissolution during ongoing weathering was observed.
However, none of these literature efforts attempted to address the issue of environmental impacts on glass properties of naturally weathered panes. There is a significant difference between glass exposed to a controlled environmental chamber (such as controlled temperature or a high humidity chamber) and the exterior environment. The external environment exposes the glass to various levels of solar irradiation, periodic condensation of water films due to humidity cycles, and the deposition of aerosol particles on the glass surface. All of these factors could have an impact on the alteration rate of glazing surfaces.
In this regard, Lombardo et al. [131] investigated the natural weathering action of SLS float glass by exposing both tin and air surfaces, unsheltered and sheltered from the rain, and examined using some well-known techniques such as SIMS, FTIR spectroscopy, EPMA, and SEM. Test results after 24 months gave evidence—and in close correlation with prior research efforts—of the uniform formation of leached layers being time-dependent, in addition to samples seemingly undergoing local corrosion phenomena. The unsheltered samples were generally more weathered than the sheltered samples, with the airside appearing more weathered than the tin side. In a more recent study, Strugaj et al. [90] studied the effect of natural weathering on two different clear float glasses exposed in three different cities with largely different environmental conditions. Optical microscopy, SEM/EDX, and AES were used to investigate surface changes and chemical compositions of the weathering products. Results from the study—after six months of exposure—generally indicated that, regardless of the glass type or weathering location, the main factors responsible for glass corrosion are humidity variations, high mean temperatures, high levels of particulate matter, and lack of rain. Solar radiation and dried water droplets were observed to accelerate the corrosion of the air side, in addition to the atmospheric attack causing irreversible changes such as delamination (chip-off of the surface of glass) within the first six months of exposure.
It is worth noting that, when several experimental literature sources are reviewed, even contradictory test results could be found for artificially weathered SLS glass [132], giving evidence of the airside presumed to be stronger and more resistant to stress corrosion. In any case, the accelerated weathering technique which utilizes a relatively high humidity and temperature methods using high temperatures and RH inhibits thorough comparisons between observation in outdoor environment due to the fact that, the effect of temperature or humidity are not strictly defined. In addition, it is also difficult to rely on any specified alteration phase and time conditions in accelerated experiments. Nonetheless, the scope of these studies did not include relating the change in surface appearance to glass strength for their otherwise carefully conducted experiments. Thus, drawing up possible lines of further research on the influence of RH on naturally weathered SLS glass.
All of these glasses described above are AN float glasses, i.e., they are not toughened glass. However, toughened glasses are now commonly employed in high-rise buildings as façade and is exposed to weathering as well as frequent mechanical and chemical cleaning procedures, which can induce alterations on glazing surfaces. Experiments related to the influence of RH on toughened glasses have been conducted in recent years, namely for leaching and layer formation [133] and influence toughening process on the mechanical performance [134].
In the case of the influence of RH on leaching and layer formation, for example, Reiβ et al. [133] carried out a comparative analysis of the surface atomic concentrations of chemically and thermally weathered toughened SLS glass using optical microscopy, XPS, and AFM techniques. Test results—after seven days of weathering in a climatic chamber at 80 % RH and 80 °C—generally showed thermal toughening has no significant effect on the surface deterioration of SLS glass. In particular, images gained by the AFM and optical microscopy (see Fig. 5), including the recorded depth profiles, inevitably verified the ion exchange given in equation (2). This trend was observed for RH values of 50 % and 100 % of SLS float glass, suggesting that the interdiffusion process (water attack) between leached sodium ions and surrounding liquid water is the same for both toughened and AN float glass. The chemically strengthened SLS glass, however, showed dramatic changes in the corrosion behavior, i.e., more pronounced lateral changes in the surface chemistry [133]. The actual behavior of such glasses—as expected from the chemical strengthening process—turned out to be closely linked to the combined change of the glass structure and a significant inhomogeneous distribution of forming crystals, thus, requiring a rigorous analysis of the combined parameters.
3.1.2. Soiling effect
Scientific inquisitiveness in airborne particulates, especially its impact on the aesthetical and visual impairment on the surface of SLS glass as well as its potential influence in the degradation of intrinsic optical properties has grown significantly over the last three decades. In so doing, estimating the soiling due to distinctive air particulates and environmental parameters such as rainfall, RH, or temperature and the influence of each type of deposit on the glass optical properties (haze, reduction in surface gloss, or light transmittance) has been documented in the literature. For instance, SEM investigations of deposited particles and haze on glass stored for six months in sheltered ambient condition reported the formation of several neo crystallization products: Na-rich sulphates/carbonates, Ca-rich sulphates, NaCl crystals, and dentric crystals, which were partially similar to species observed in the local precipitation or immediate environment (see Fig. 6) [81]. As such, when addressing glass degradation, the deposits and reactivity of particulates with/on the glazing surfaces should be properly taken into account.
The effect of different levels of airborne particulates (SO2 and NO2) combined with RH and temperature on the soiling process of SLS glass both at ambient (without pollutants) and in an RH chamber was investigated by Cummings et al. [135] by means of Rutherford backscattering and NRA. (exposure time up to 400 h, RH ranging from 10 to 100 %, temperature fixed at 40, 70, and 90 °C, SO2 concentration of about 11 ppm). In General, glass corrosion was found to be substantially higher on specimens exposed to pollutants, than on glass exposed to the ambient environment. This impact could be induced by soluble elements in the particulates, and it could be more proof of the corrosion susceptibility of this type of glass in polluted environments, and particularly for dusty urban or industrial atmospheres. However, there was no indication that the two pollutants interact to give a synergistic effect. RH values, on the other hand, had a remarkable impact on the hydration rate than the levels of SO2 or NO2, implying that moisture is the main weathering factor accounting for the corrosion and leaching phenomenon in SLS glasses.
The natural outdoor exposure of comparatively durable SLS AN glass in polluted background locations in six European municipalities was reported in [136]. Test observations after 2 years of exposure indicate weight gains in glasses exposed in sheltered environments as a result of deposition of APM, while weight loss was reported for specimens exposed unsheltered, presumably as a result of leaching or dissolution of the weathering particles by precipitation on the glass surfaces. The source of a vast percentage of the surface deposits was also found to be anthropic, probably resulting from diesel cars exhaust emissions. Upon two years of exposure, the leached layer profile of Na analyzed by SIMS revealed only mild leaching up to a depth of 40 nm of this glass type. Inference from the DRF relations characterizing the surface soiling between the main optical parameters and time indicated a steady increment—approximately 50 % of the saturation level—in the soiling rate during the first nine months, due to the excellent receptivity of the originally cleaner glazing. A decline in the soiling rate was noticeable at later times, probably due to the APM deposits emerging as a second layer [136]. The progression of both deposition and optical parameters could be inferred to have followed the same responsive pattern expressed in Equation (2).
In 2021, Strugaj et al. [90] exposed two different types of float glasses (white and greenish colourations) to urban, rural, and seacoast locations. The authors gave evidence of delamination effects and iridescent films—considered as the most severe attack observed on the examined glass—being most frequently detected on the samples weathered in the seacoast environment. These effects of atmospheric attack could be intensified by the high and homogenous factors such as marine aerosols, sand dust, high temperatures, and lack of rain. In contrast, specimens exposed in urban and rural environments experienced more frequent snow and rain than those in the seacoast areas which enabled these specimens to maintain a cleaner top side, indicating that the soiling deposition rates maybe differ significantly from one location to another. Regardless of the glass type or specimen sides (air/tin), significant deposition differences were not observed, suggesting longer exposure times for such type of tests. Studies pertaining to the soiling effect of toughened SLS glasses could not be found in the literature, hence, drawing up possible research on these glass types.
3.2. Environmental impacts on glass mechanical properties
The accumulated damage and subsequent degradation of structural glass systems is due to the natural ageing induced by environmental conditions and the degree of damage varies depending on the duration and level of exposure. As a result, when designing and constructing with glass, it is imperative to understand its long-term mechanical behavior. Comparisons of experimental efforts so far indicate notable differences between new glass plates and those exposed to in-service conditions. However, very few experimental studies on the mechanical performance of naturally aged SLS float glasses exist in the literature and far fewer on the mechanical performance of naturally aged tempered glasses. In this regard, numerous experimental studies have focused on the artificial weathering of SLS glass, in order to characterize the weathering effect on its surface and relate the damage and surface alteration to strength reduction. In the following sections—particular emphasis on recent methods and result—we aim to highlight the disparities to identify the peculiarities of glazing alterations due to weathering and outline potential direction for future studies.
3.2.1. Effect of natural outdoor exposure
The earliest study which relates to the strength of naturally weathered glass specimens was presented by Abiassi [137], who reported experimental strength test results on SLS glass from three different buildings having approximately 25 years of weathering and in-service exposure and to make a direct comparison to the strengths of new glass plates. Test results collected in [137], showed that the weathered window glass suffered up to 50 % loss in strength compared to the new window glass. A realistic comparison was made by taking advantage of the glass failure prediction model (GFPM) [138]. The model showed that the in-service window glass obtained from the three different buildings generally exhibited the same probability of failure as the new glass plates, providing a cross-correlation with Beason, wherein it was observed that glass strength significantly deteriorates after 20 years of in-service conditions.
Norville and Minor [139] also reported the results of destructive tests conducted on weathered window glass plates under uniform lateral pressure. Weathered float glasses (inside and outside)—502 × 1727 × 5.82 mm, their nominal sizes—with eight years of in-service conditions were considered. Also included in the tests series were coated and uncoated IG units (weathered) and new glass plates. Test results generally showed the exterior surfaces of the IG units were more weathered than the interior surfaces. A negligible decrease in strength was observed in the IG units when compared to as-received glass specimens. This could be attributed in part to the coating of the IG units. However, these studies did not evaluate the impact of RH or temperature on the strength deterioration of naturally aged plates.
The mechanical performance of aged-toughened glass can be evaluated by testing naturally aged specimens. However, sourcing naturally aged-toughened glass is difficult due to the relatively recent use of toughened glass in the building industry. Studies pertaining to the behavior of toughened glass exposed to the natural outdoor environment are far fewer in the literature, in fact, only two studies seem to exist on the natural ageing of FT glass. In 2016, Afolabi et al. [140] performed an experimental analysis with weathered FT monolithic glass specimens. Virtual examination (showing notable surface damage such as scratches on both sides) was conducted on fourteen specimens—1520 × 740 × 6 mm their nominal size—and subjected to destructive load testing. In general, it was observed that the FT weathered glazing exceeded the ASTM [141] strength prediction after 20 years of exposure, suggesting that FT glass does not weather like ordinary AN float glass. However, a comprehensive statistical analysis of the original strength data of the glass 20 years ago was not reported.
Later on, M. Yussof et al. [142] conducted short-term monitoring on FT glass, by exposing specimens (1100 × 360 mm their nominal size) to the outdoor environment. Test results after 120 days—12 specimens in total—however, generally showed a noticeable decrease in the bending and residual strengths of specimens with increasing weathering duration, with suggestions of an increase in the intensity of surface defects due to environmental conditions as exposure time increased. No other such study on naturally weathered FT glazing could be identified, hence requiring further detailed investigation of this glass type.
3.2.2. Artificial ageing
The artificial ageing technique, which has been largely aimed at evaluating the deterioration in the optical transmission properties of SLS glasses, has attracted interest over the last decades for evaluating the mechanical performance of weathered glazing systems. This technique is considered a valuable tool in assessing the mechanical durability of aged glass by utilizing aggravated conditions such as sand abrasion and scratching mechanisms to speed up the natural ageing process to assure its continued usage in load-bearing applications. However, DIN 52,348 [143] which proposes a falling abrasion technique, is presently the only guideline in characterizing the mechanical performance of artificially aged glazing systems. See, e.g., [144], [145].
In these studies, the erosion resistance, however, was primarily assessed using non-destructive tests (i.e., mass loss, optical transmission, and roughness characterization), thereby discounting the strength of the glass. Although basic strength data is presented in [146], [147], detailed statistical analysis on the strength of glass is only presented in [148], which reported a 59 % decrease in the characteristic strength of as-received glass after sand trickling with 6 kg of sand dropped from a height of 1 m. The actual performance from these type of tests—as expected from the examined boundary condition—turned out to be specifically dependent on the mass of sand and impact velocity. Hence, more detailed investigations on the sand abrasion technique are required to evaluate its effect on the artificial aged glazing systems and its correlation to their natural weathered counterpart.
In this regard, Datsiou and Overend [149] investigated the efficiency of the sand abrasion technique for the artificial weathering of annealed glasses. By adopting varying combinations of weathering factors, the authors compared the strength of naturally aged AN (20 years of in-service condition) and new AN glass plates (150 × 150 × 3 mm their nominal sizes). Images obtained from optical microscopy of glass surfaces showed mild to extensive flaws on the naturally weathered glass panes. Damage to the artificially aged specimens was also found to be dependent on the size of sand grains (Fig. 7, Fig. 8). Results from coaxial double ring tests generally showed that the natural weathered glasses decreased by roughly 85 % in their design strength compared to as-received glass. However, it was observed that DIN 52,348 [143] significantly overestimated the strength at lower probability of failure in comparison to the natural weathered AN glasses (see Fig. 9). Perhaps the most notable observation from the study is that the degree of defect incurred by the falling abrasive method proposed in [143] appears to be arbitrary and does not correlate well to the observed performance in the natural weathered glasses [149].
![Fig. 7. Optical micrographs of tested specimens (a) – (b) naturally weathered, (c)as-received glass, (d) artificially aged glass with 0.7 mm grain size sand, (e) artificially aged glass with 5.6 mm grain size sand, (f) artificially aged glass with 9.5 mm grain size sand [149].](/sites/default/files/inline-images/Fig7_242.jpg)
![Fig. 8. Surface defects observed in (a–b) naturally aged glasses, (c–d) artificially aged glasses with 0.7 mm grain size sand, (e) artificially aged glass with 5.6 mm grain size sand, (f) artificially aged glass with 9.5 mm grain size sand [149].](/sites/default/files/inline-images/Fig8_209.jpg)
![Fig. 9. Comparison of test results for as-received and naturally weathered vs artificially weathered with sand abrasion (a) Probability of failure, DIN 52348, (b) Probability of failure, best performing SA series, (c) Mean strength, and (d) Design strength [149].](/sites/default/files/inline-images/Fig9_185.jpg)
Same authors later used a similar ageing technique to evaluate the strength decrease in SLS AN, SLS fully toughened glasses by producing surface defects comparable to 20 years of natural ageing [150]. Chemically toughened aluminosilicate glass was also incorporated among the tests series. Weathering of all glass studied—as expected from the examined boundary conditions—was indeed associated with a loss in strength compared to as-received glass. It was also observed that the FT glass provided a better post-aged performance. However, the degree of toughening was also said to have affected erosion resistance, giving evidence—consistent with prior studies [151], [152]—of the relatively high hardness of chemically toughened glass.
Moving from erosion resistance to scratch resistance, it is worth noting that, when several experimental literature sources are reviewed, even contradictory test results could be found, casting doubts about these techniques and its potential in generating realistic defects. In fact, different ageing methods have been shown to be preferable for two different types of natural weathered glasses, after exposure to erosive action and linear scratching [150]. In the study by Datsiou and Overend [153], in particular, the inducement of scratch on SLS AN glass (150 × 150 × 3 mm their nominal sizes) was favored over all other abrasion techniques—providing evidence of scratching providing decent optical fit—based on dye penetrant used for revealing surface defects in the naturally aged glass. In addition, artificial ageing with the erosive abrasion technique was quite challenging to replicate. In contrast, SLS AN glass which was artificially aged using the sand trickling technique was observed to be better reflective of the strength and surface roughness as the referenced naturally aged AN glass than that of scratched glass [152]. Notwithstanding present researches on scratch and erosive techniques of SLS glass, a detailed and viable technique for the artificial weathering of structural glazing systems has yet to be determined, hence, necessitating more experimental testing and analysis.
In the case of LG systems, for example, the effects of rheological parameters such as temperature or direct sunlight or RH levels which poses degradation issues in commonly used interlayers, should be adequately considered. In this regard, Centelles et al. [44] conducted an experimental analysis on the bond behavior of various polymeric materials—PVB, Saflex, SG, EVASAFE—commonly used as interlayers in laminates. Both environmental actions—humidity, thermal cycles, and solar radiation, in environmental chambers—and mechanical tests were carried out on the LG units (10 mm thick AN glass with 1.52 mm thick interlayers). Results from the study gave evidence—and in accordance with prior literature findings [43], [154]—of a loss in adhesion in PVB specimens on exposure to humidity conditions, as well as producing a stiffer material after exposure to UV and thermal cycles. EVASAFE and SG specimens, however, showed better resistance performance to weathering actions.
Results from the double-lap shear test indicated that, dependent on the mechanical properties of each polymer and its level of adhesion with the glazing unit, there could be glass fracture—as observed in the PVB and SG specimens—or adhesion loss between interlayer and glass accompanied by subsequent glass fracture, which was mostly observed in the EVA specimens. The study concluded that SG and PVB with minimum plasticizer are the best choices for LG units in structural applications due partly to their maximum stresses and also high initial stiffness when not exposed to humidity conditions. The authors, however, emphasized that the performance of polymeric materials for LG elements may differ after the laminating processes and this could make pointless, over time, precise numerical analyses of the mechanical responses of laminated structural elements. Hence, requiring more dynamic experiments at various temperature levels to investigate the thermo-viscoelastic behaviour of these polymeric materials.
Although several experimental studies have focused on the degradation of LG panes and interlayers as a constructional material due to environmental stresses, limited literature efforts are still available on the outdoor natural weathering of laminated glass (e.g., see a selection in Table 4). As far as the authors know, outdoor weathering of LG elements has been performed only by Ensslen [45]. The author reported an extensive experimental evaluation on the behaviour of circular LG units—100 × 100 mm and 300 × 300 mm their nominal sizes—glued together by PVB foils and subjected to environmental stress conditions. Some samples were exposed to weathering processes in humidity and temperature mixed conditions in a climatic chamber, another set of samples were exposed to UV radiation in a solarium, while others were simply exposed to the natural outdoor environmental conditions for up to two years at various climatic locations.
Monotonic shear test was used to compare specimens that had been weathered artificially to those that had been subjected to the outdoor environment. In general, results from the study demonstrated large increments in moisture absorption of specimens artificially aged but no significant increase in moisture absorption of naturally weathered specimens. Shear test results indicated a stiffer and more brittle PVB foil, as well as adhesion loss and delamination at the edges of LG units could also be observed in artificially aged specimens. The naturally weathered specimens, on the other hand, showed only minor derivations in overall shear performance. However, at low test speeds, delamination could also be observed at the edge of specimens naturally weathered in extreme climatic conditions. The actual behaviour of such glasses—as expected from the test conditions—turned out to be closely related to local instabilities i.e., buckling, thus, requiring more rigorous experimental analysis of naturally weathered LG units and systems.
Table 4. Environmental impacts on structural glass: summary of selected experimental research studies.
4. Retrofitting and strength enhancement of weathered structural glass
The application of protective coatings is an alternate solution to the well-known traditional tempering processes for glass strengthening. Many varieties of protective coatings that can enhance the mechanical performance of glass windows are available on the market. Study findings have shown that the mechanical performance of structural glazing could in fact be improved using these protective films [155], [156], [157]. However, different authors have attributed the strength enhancement to either physical, chemical, or thermal phenomenon. For instance, Fabes and Berry [158] were one of the first to give scientific proof of the high potential of protective coatings for glazing systems, carefully supporting a physical explanation for the increased performance of windows based on the controlled flaws induced on SLS AN glass. The authors ascribed the enhancement to the partly infill and subsequent decrease of the assumed cracks, such that the crack-face was practically an embedded defect, taking a differing form compared to the initial glass surface.
A related mechanical explanation for the apparent increase in performance was later provided in [159], based on the bridging influence of the injected coating on the induced surface crack. The technique required the application of a relatively tiny layer of the protective film on the crack tip, which, as a result of the effect of Poisson ratio, impeded the cracking growth in the ratio of the entire glazing. An alternate strengthening process is ascribed to the thermal expansion disparity between the protective coating and studied glazing. As reported by Hand et al. [160], this technique produces healing stresses inside the generated defects. Another strengthening process may be chemical, in which the coatings protects the crack tip from RH. This mechanism eliminates or reduces the deteriorating impact of the stress corrosion phenomenon or the sub-critical growth crack, which has been observed to emerge in moist conditions or in the presence of tensile stresses [161].
These study findings provided a valuable body of knowledge on the strengthening effect of resins on weathered glass. For instance, a majority of these experiments are in agreement that the penetration depth of these resins inside the crack tip is a significant factor in the strength recovery process. Nonetheless, there are still some complexities within this area, such as, the strength restoration results were mainly portrayed and analysed with respect to mean values, which is a convenient correlation in itself, however, it is not practical in structural applications where lower failure probability dictate its evaluation. In addition, most of these retrofitting methods appear fixated on resin repair techniques on artificially damaged glass, which may preclude their use on naturally weathered windows. Thus, a detailed assessment of both artificially damaged and naturally weathered resin-based repairs is required.
In this regard, in 2018, Datsiou et al. [162] investigated the strength recovery of artificially aged AN glazing by taking into account 150 × 150 mm SLS glass (3 mm their nominal thicknesses) after the volumetric filling of visible flaws on the glass surface using three repair techniques involving (i) acrylic resins (ii) polishing (iii) acid treatment. Coaxial double-ring tests were conducted at inert conditions, in contrast to previous experiments on protective resins that adopted the four-point bending test method at ambient lab conditions. The study gave evidence of the polishing technique being a better fit, indicating good strength recovery data after subjecting specimens to various environmental ageing tests (freeze–thaw, humidity, and UV cycles) (see Fig. 10). However, similar strengthening effects have not been reported, drawing possible lines for further research. The polishing repair—notwithstanding it provided better efficiency—was incapable of achieving full strength recovery by 25 and 51 % at mean and design probability of failure, respectively, when compared to as-received glass.
![Fig. 10. Strengthening of artificially aged glass (a) resin application, (b) pressure injection, forcing resin into the flaws, and (c) CDR plots for polish strengthened glass after UV irradiation [162].](/sites/default/files/inline-images/Fig10_156.jpg)
5. Concluding remarks and future work
In many applications, glazing elements are repetitively exposed to weathering as well as frequent mechanical and chemical cleaning procedures, which can lead to changes in the glass surface. Hence, the efficiency of the products decreases with age. Such deterioration and ageing processes are mainly determined by various environmental conditions such as relative humidity, temperature, solar radiation, and air-borne pollutants.
This paper presents the assessment of SLS glass performance against such ageing scenarios based on available literature findings. Only experimental studies are included in the comparison to make it more practical and consistent. The weathering resistance of several categories of structural glass systems is sequentially presented. Toughened, laminated or multi-pane glasses are suggested for use in engineering applications due to their superior weathering resistance in comparison to ordinary AN float glazing. However, judging by the market pricing report, these glazing systems are generally more costly than AN glass. In addition, glasses like multi-pane or even laminated glass will arguably add to the weight of the façades. Hence, extra criteria should be taken into account when choosing a glazing type.
By reviewing the major experimental works, several remaining questions are summarized from the authors' viewpoint. The tremendous advancement in technology within the glazing industry is already witnessing rapid growth in the production and supply chain of innovative glazing products in the glazing market. Irrespective of the glazing type, when these glasses are exposed to environmental conditions, damages will be accumulated on the glazing surfaces throughout its service life. The weathering effect on its mechanical performance should be continually investigated in order to gain better knowledge of glasses under environmental actions. In addition, artificial ageing techniques currently have a great deal of inconsistency in estimating the long-term mechanical properties of glazing elements, owing to the lack of an artificial ageing mechanism consistent with the real-world accumulated damages. Thus, accurate artificial ageing models are needed to be devised to aid in the prediction of weathering resistance and risk assessment in structural glass applications. In light of these considerations, further monitoring and tests of naturally weathered SLS glass are still required to ascertain the long-term impact of these environmental factors.
Also, there has been much less discussion on temperature effects, and the impact of environmental temperature on the properties of laminated glass interlayers is still not accounted for precisely in the literature. Different researchers agree that solar radiation increases the surface temperature of glass in a way that promotes chemical alteration and can also induce thermal stress. However, more detailed experimental tests are required to investigate the damages produced by environmental temperature on LG systems since thermal variations may have quite diverse impacts on the various types of interlayers, depending on the transition temperature of each interlayer. The findings reported by different authors do not appear to be indicative of this phenomenon.
However, more detailed experimental tests to investigate the damages produced by UV radiation on various LG interlayers are required, so as to identify possible diverse deterioration mechanisms in these interlayers.
Lastly, in recent years, research attempts have been made in evaluating the efficacy and reliability of specialized protective resins and coatings for rehabilitation and safe guarding existing window glass and structural glazing system in general. Following earlier observation, these protective coatings typically demonstrated significant improvement for uncoated glazing elements, however, more research is needed to fully achieve their potential.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors acknowledge with gratitude the financial support provided for this study by the Ministry of Higher Education Malaysia in the form of Fundamental Research Grant Scheme, FRGS/1/2018/TK06/USM/02/3.