The comparative evaluation of vacuum evaporated chromium thin film adhesion strength to float glass treated under different surface preparation methods was performed using scratch tests. Also, the influence of float glass substrate heating for chromium thin film adhesion strength was investigated.
Surface activation and contamination removal by O2 plasma process and RCA-1 surface preparation method revealed the best results, where surfaces were free of hydrophobic contaminants with low contact angles ranging from 4° to 8°.
For majority of float glass surface preparation methods the contact angles were observed slightly higher for the “tin side” as compared with “air side”. Soda-lime-silica float glass surface wettability properties of both sides were equalized during bombardment in RIBE unit by Ar+ ion beam.
O2 plasma process and RCA-1 surface preparation method for float glass resulted in a higher value of the thin chromium film adhesion to the float glass substrate as measured from the average critical normal force (O2 plasma 0.169 N; RCA-1 0.14 N) in scratch tests. The float glass substrate heating (100 °C) in the vacuum chamber before chromium thin film evaporation process resulted in even higher value of the thin chromium film adhesion to the float glass substrate.
Keywords: float glass, contact angle (CA) measurements, thin chromium film, scratch test, adhesion.
Algirdas LAZAUSKAS, Viktoras GRIGALIŪNAS
Institute of Material Science of Kaunas University of Technology, Savanorių 271, LT-3009 Kaunas, Lithuania
Tel.: + 370-671-73375; fax.: +370-37-314423.
E-mail address: algirdas.lazauskas@stud.ktu.lt (A. Lazauskas)
1. INTRODUCTION
Chromium thin film on glass substrate is an important material for the lithographic photomask production as well as in production of diffraction gratings or precision glass scales for photoelectric measurement systems [1]. Among many other applications, chromium thin films are also used in surface micromachining technology as interlayer for improvement of adhesion [2].
The thin films can be deposited by various techniques, including e-beam evaporation [3], thermal evaporation [4], sputtering methods [5 – 6], etc. Whatever their intended use may be, the properties, structure, functional characteristics, and performance all depend, inter alia, on adhesion between the thin film and the substrate [7]. A clean, dry substrate surface is a necessary prerequisite for adhesive bonding.
Rarely can a structural adhesive penetrate through surface contaminants to provide an optimum bond on an unclean surface. Therefore, surface preparation is essential for manufacturing high-quality glass substrate based products coated with thin films.
Surface preparation methods can be grouped into two categories: wet chemical cleaning involving solvents, and dry cleaning involving ions, electrons, free radicals, and other neutral species. Prominent examples of wet chemical cleaning processes for glass substrates are RCA-1 clean, RCA-2 clean and piranha clean. RCA-1 and piranha are used as a glass surface preparation methods for removing organic residues and certain metals [8 – 14]. RCA-2 forremoving atomic and ionic contaminants.
If wrongly applied, cleaning is replaced by etching and from the aqueous cleaning processes a surface roughening can result [10]. For large volumes of glass substrates an ultrasonic cleaning with aqueous solution containing detergent and surfactant is used. It is effective in removing large contaminants but removal of micro contaminants requires greater mechanical energy e. g. high velocity water jet cleaning process [15].
For modification of glass surface chemistry, dealkalization processes of exposure of glass substrates to atmospheric humidity (also known as weathering) or hot deionized (DI) water (also referred to as leaching) for a period of 3 – 4 days are used [16]. A typical example of dry glass surface preparation method is plasma treatment [17 – 18]. Plasma treatment can be used for surface activation and contamination removal.
Surface activation is a process where surface functional groups are replaced with different atoms or chemical groups from the plasma utilizing source gases such as oxygen, hydrogen, argon, or a mixture of these gases. Surface contamination (i. e. residual organic solvents, oxidation, epoxy residue and etc.) removal by plasma is an ablation process, where physical sputtering and chemical etching are the key processes involved [19].
Oxygen plasmas create an acidic, while nitrogen produces an alkaline chemistry. Oxygen plasmas are very effective in oxidizing residual hydrocarbon and other organic contamination. Argon plasmas are useful in decomposing and desorbing organics [20].
Float glass is used as a substrate in microelectronic, mechanical systems or “lab-on-a-chip” applications because of its excellent chemical durability and inexpensiveness [21]. Float glass has excellent flatness over a large area without polishing and the productivity is extremely high. 90 % of the world’s production of flat glass is formed using float technology [22 – 23]. Molten glass is poured onto a liquid tin bath, on which it cools and solidifies to produce two flat and parallel surfaces of a sheet. The surface in
contact with the molten tin bath experiences tin diffusion and is referred to as the “tin side” while the top side, often called the “air side”, can experience surface dealkalization because of the surrounding environment [24]. The tin side is also in contact with supporting rollers during heat treatment. After solidification, the continuous glass sheet undergoes a carefully controlled cooling process to eliminate any residual stresses before being cut into sheets for subsequent use. Thus the two sides of the glass sheet undergo different histories during processing [25].
With respect to the wettability of the glass surface the contact angle is of interest. In general, the lower the contact angle the higher the surface energy. The increase of energy and decrease of contact angle usually correlates directly with improved adhesion. For the glass substrates contact angles (water) change in the range 34° – 36.5° as reported in [26] and [27].
After application of the RCA-1 clean the glass surface wettability is increased, displaying low contact angles 2° ±2° as observed in the previous studies [10] and [28]. The piranha clean treatments on glass resulted in highly variable contact angles ranging from <8° to 43° in [28], 4° in [13] and 21° to 27° in [14]. Plasma treatment on glass with oxygen reaction gas even at short exposure time resulted in contact angles to be below 10° as it was reported in [29] and [30].
Previous work [14] reported that there was no correlation between surface roughness and contact angle hysteresis, contact angles, or dewpoint error for glass surfaces. Surface preparation methods on glass had similar contact angles but significantly different surface roughness. Therefore in this work roughness effect on surface preparation methods have not been examined.
The aim of this work was to clarify previously conducted studies of surface preparation methods for glass and their effect to surface wettability in terms of contact angle measurements. The second aim was to conduct comparative evaluation of vacuum evaporated chromium thin film adhesion to glass treated under different surface preparation methods using scratch test. Also, the influence of substrate heating for chromium thin film adhesion was investigated.
2. EXPERIMENTAL TECHNIQUE
A commercial soda-lime-silica float glass of thickness 1 mm was used in this study. The chemical composition by weight for the clear float glass was provided by manufacturer (Pilkington NSG Group Flat Glass Business)and is as follows: 72.6 % SiO2 , 13.9 % Na2O, 8.4 % CaO, 3.9 % MgO, 1.1 % Al2O3, 0.6 % K2O, 0.2 % SO3, 0.11 % Fe2O3.
The glass was obtained without any acid interleave coatings on their surfaces. Since two sides of the glass sheet undergo different histories during processing, their composition and properties of the surface differ. Therefore both the tin and air sides were examined for the soda-lime float glass. Observing the fluorescence under UV illumination easily identified the tin side of float glass.
Glass was cut into (3 × 3) cm² slides. The air and tin sides of the float glass were subjected to nine surface preparation methods in order to remove debris, possible reaction products, organic contaminants that might exist on the surfaces.
Surface preparation methods included: rinsing with deionized (DI) H2O and drying under compressed O2 (chosen as control method); processing using ion-plasma preparation method: the slides were exploited in O2 plasma in the camera of the device “Plasma-600 T” with the presence or 133 Pa pressure (RF = 13.56 MHz, P = 0.3 W/cm²); ultrasonic cleaning in acetone for 10 min; leaching: glass slides were immersed in 50 °C DI H2O for a period of 5 days; RCA-1 clean: a batch of RCA-1 cleaning solution (6 parts DI H2O, 4 parts 27 % ammonium hydroxide NH4OH, 1 part 30 % hydrogen peroxide H2O2) was prepared and heated on a hot plate until it bubbled vigorously.
The slides were then immersed in this solution for 60 min with continued heating and stirring. Subsequently, slides were rinsed 3 times with deionized water and dried under compressed O2; bombardment in RIBE unit (“Usi-ionic”) by Ar+ ion beam (etch rate ~40 Å/min) using multicell cold hollow-cathode DC ion beam source (Ar+ ion energy 300 eV, ion beam current 0.25 mA/cm², pressure 7 × 10¯² Pa, substrate temperature 293 K ±5 K); Piranha clean: a batch of Piranha cleaning solution (2 parts concentrated 96 % sulfuric acid H2SO4, 1 part 30 % hydrogen peroxide H2O2) was prepared and heated until it bubbled vigorously.
The glass slides were left normally for 30 min in the solution, after which they were removed rinsed 3 times with deionized water and dried under compressed O2. The summary of surface preparation methods used in this study is presented in Table 1.
Table 1. Surface preparation methods used in this study

Wetting phenomena have been studied scientifically during the past 200 years, to one of the many achievements of the British scholar Thomas Young [31]. He suggested a simple equation that equilibrates the forces at the contact point of a liquid drop on a solid surface [32],
![]() (1)
(1)
where Y denote the excess free energy per unit area of the interface indicated by its indices g, l, and s corresponding to the liquid, gas and solid, respectively. The liquid/gas excess free energy Y l,g , corresponds to the surface tension of the liquid with its vapor. Contact angle θ, which is the other experimentally easily accessible factor in Young’s equation is even more important to this paper. Contact angle measurements allow general comparisons and can provide a rapid qualitative test for surface wettability characteristics [28].
Contact angle measurements were performed at room temperature (20 °C) by the sessile drop method. One drop of deionized water (5 μl) was deposited onto a dry glass surface. Images of drops were magnified, photographed, and contact angle measured using method based on B-spline snakes (active contours).
The method offers the best tradeoff between the use of the general drop shape to guide the detection of the contour of the drop, and the use of an algorithm with local behaviour to compute contact angles with high-accuracy [33]. The method is made freely available as DropSnake plugin [34] for ImageJ, which is a free open-source multi-platform Java image-processing program [35].
Three measurements were used to evaluate each surface preparation method for air and tin sides of the glass. Each reported value is the mean contact angle of three measurements ± standard error of means. Measurements of contact angles were taken within 10 s after formation of the sessile drop.
Chromium thin film deposition was performed using a thermal evaporation technique. Vacuum in the chamber (turbomolecular pump) was 10¯³ Pa. Approximately 50 mg of >95 % pure chromium chips (1 mm to 4 mm diameter) were placed in the tungsten boat, which was located 100 mm below the substrate. The tungsten boat was heated approximately up to 1656 K (corresponding 1 Pa solid state chromium vapor pressure) using an electric current. Substrate heating (100 °C) was performed with IR lamp heater installed in the vacuum chamber.
The substrate temperature was controlled with “K” type thermocouple probe contacting the substrate. The thickness of chromium films 40 nm was controlled with quartz crystal deposition controller.
The scratch test has been used widely for evaluation of film/substrate adhesion [36 – 38]. At present, quantitative adhesion data from the test is difficult to extract. In most cases the stresses around the moving indenter are too complicated to be predicted accurately and therefore the stresses driving coating failure are not known. However, the scratch test is a good method for quality assurance/quality control testing of the adhesion of hard films and is useful in the development of new coatings for process optimisation [39].
The adherence of thin chromium films was measured with custom-made PC controlled scratch testing apparatus. During the scratch test the glass slides with chromium thin film were scratched with an indenter applying the increasing normal force (load). The displacement of the indenter, normal force and the occurring tangential (frictional) force were recorded. The abrupt change in tangential force pinpoints the spot on a scratch track where the chromium layer starts to flake and gets detached from the float glass substrate, i. e. at the critical normal force [40].
Afterwards glass slides with chromium thin film were examined under an optical microscope in search of characteristic defects. The chromium thin film adhesion to float glass treated under different surface preparation methods were evaluated by comparing critical normal forces of film delamination.
Five scratches were performed on each glass slide with chromium thin film and average values of critical force were calculated. Either two or three critical loads were determined for each slide by optical microscopy inspection. The principal scheme of acting forces and force moments of custom-made PC controlled scratch testing apparatus is presented in [41].
3. EXPERIMENTAL RESULTS AND DISSCUSION
Surface preparation methods that effectively remove debris, possible reaction products and organic contaminants from the float glass surface produce a very hydrophilic surface with low water contact angles. Conversely, methods which incompletely remove organic contaminants will leave a more hydrophobic surface with higher contact angles. To ensure reproducibility, at least three measurements were carried out for air and tin sides of the glass. Since contact angles were constant during each run, they were averaged to yield a mean value for the experiment.
Fig. 1 presents mean contact angles of air and tin sides of float glass after different surface preparation methods (Table 1).

Fig. 1. Mean contact angles of air and tin sides of float glass after different surface preparation methods (Table 1). Each bar represents the mean of three measurements per slide. The standard error of the mean for all contact angles was less than 1º
As shown in Fig. 1, the contact angles obtained by control method were obtained by ~10° lower comparing to as-obtained glass in [26] and [27]. Methods 2 – 4 and 7 revealed the best results, where surfaces were free of hydrophobic contaminants with low contact angles ranging from 4° to 8°. Here, the organic contamination resulted to be no greater than several percent of a monomolecular layer in coverage and contact angles were close to surface total wettability.
Comparing with control method (Method 1) it is assumed that oxygen plasma treatment (Methods 2 – 4) activated glass surface causing the decrease of contact angle from 20° ±2° to ~6° due substitution of chemically active dangling bonds and hydroxyl groups for the carbon contamination in the surface resulting in decreased oxidation as it is reported in [29, 30]. It is considered, that the use of Method 7 is also attributed to leaving hydroxyl groups on the glass surface, imparting negative charge to the surface (making it hydrophilic) [10].
The contact angles obtained by Methods 2 – 4 and 7 are close to those reported in the literature. Surface activation and contamination removal by O2 plasma process require the investment in proper equipment. It has the advantage of being able to clean considerably more glass slides at the same time with little or no waste products requiring less operator input than wet chemical cleaning procedures and is more suitable for industrial applications. The alternative RCA-1 surface preparation method gives practically the same soda-lime-silica float glass surface wettability properties as plasma treatment and is being easy to set up, requiring the basic chemicals and the use of a fume hood.
However, it generates toxic waste products and is more suited in small research laboratories. For Methods 1 – 7 the contact angles were observed slightly higher for the tin side of float glass. The previous work results [42] suggests that the tin side of soda-lime-silica float glass surface OH group density is higher than the air side resulting in higher adsorption of organic substances in the atmosphere, and thus governing the wettability of the float glass surface. In order to clarify these results the Method 8 was used where contact angles for tin and air sides of float glass were obtained basically equal.
Thus, from [42] we consider that Sn present monolayers of tin side in our case were etched during bombardment in RIBE unit by Ar+ ion beam thus equalizing glass surface wettability properties of both sides. The contact angles obtained using Method 8 are nearly the same as reported in [14]. The Piranha clean of Method 9 resulted in opposite reaction, as shown in Fig. 1.
It is considered that surface heterogeneity caused by the leaching and/or etching actions of the cleaning agent differently affected float glass surface wettability properties as compared with Methods 1 – 7. The contact angles obtained were rather similar with results obtained in [14] than with [13]. Also, it is considered that the leaching actions of Method 5 and ultrasonic clean in acetone (Method 7) did not noticeably alter soda-lime-silica float glass wettability properties.

Fig. 2. Tangential and normal force as a function of displacement: 1 – Cr/glass slide, where float glass surface was prepared using Method 1, critical normal force is 0.087 N; 2 – Cr/glass slide, where float glass surface was prepared using Method 4, critical normal force is 0.169 N; 3 – Cr/glass slide, where float glass surface was prepared using Method 7, critical normal force is 0.14 N
In Fig. 2, the applied normal load and resulting tangential force are displayed as a function of displacement for Cr/glass slides, where soda-lime-silica float glass surface was prepared using Methods 1, 4 and 7. In all plots, the first part shows a linear behavior of the tangential force with respect to the applied load. The change in tangential force is detected at the critical normal force where coating failure occurs. For glass slides prepared under Method 1, the average critical normal force is 0.087 N, whereas it is 0.169 N and 0.14 N for Methods 4 and 7. It can be seen that critical normal force decreases with increasing contact angle.
Slight variations of critical normal force were observed in all measurements for tin and air sides of float glass. The highest average critical normal force was observed for Cr/glass slides, where sodalime-silica float glass surface was prepared using Methods 2 – 4 and 7. It follows that O2 plasma process and RCA-1 surface preparation method for float glass results in less contaminated surfaces and a higher value of the thin chromium film adhesion to the float glass substrate as measured from the critical normal force in scratch tests. The summary of the scratch test results is presented in Table 2.
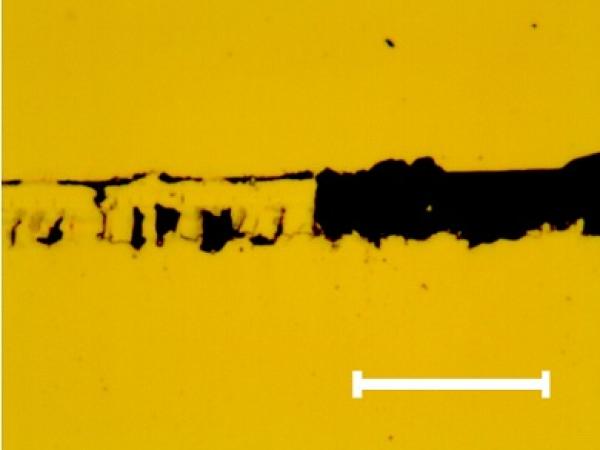
The optical analysis at the positions at which the change in tangential force was observed showed the characteristic images of chromium thin film delamination (see Fig. 3 for characteristic image of full delamination).
Another series of float glass slides prepared using Method 4 were subjected to heating (100 °C) in the vacuum chamber before chromium thin film evaporation process. The scratch tests revealed no change in tangential force over all range of scratch track. It can be concluded that the average critical normal force for coating delamination for these samples exceeds 0.2 N. This was also confirmed by optical analysis of the produced scratch track. No stripping or coating failure was observed.
Table 2. Summary of the scratch test results, average critical normal force of Cr/glass slides, where soda-lime-silica float glass surface (air side and tin side) was prepared using different surface preparation methods (Table 1)


Fig. 3. Characteristic optical image of the scratch track section where the full delamination of the chromium thin film occurs. The same fracture can be observed on the other samples. Mark size 10 μm
4. CONCLUSIONS
Surface activation and contamination removal by O2 plasma process and RCA-1 surface preparation method revealed the best results, where surfaces were free of hydrophobic contaminants with low contact angles ranging from 4° to 8°. From these results, it is clarified that oxygen plasma treatment and RCA-1 clean activates the float glass surface, makes the surface hydrophilic and increases the wettability.
For Methods 1 – 7 the contact angles were observed slightly higher for the tin side of float glass. Soda-limesilica float glass surface wettability properties of both sides were equalized after bombardment in RIBE unit by Ar+ ion beam. From these results, we assume that the tin side of soda-lime-silica float glass surface OH group density is higher than the air side resulting in higher adsorption of organic substances in the atmosphere, and thus governing the wettability of the float glass surface.
It is considered that the leaching actions of Method 5 and ultrasonic clean in acetone (Method 7) did not noticeably alter soda-lime-silica float glass wettability properties.
The oxygen plasma treatment and RCA-1 clean was clarified to be an effective method for improved adhesive bonding properties.
Float glass substrate (prepared using Method 4) heating (100 °C) in the vacuum chamber before chromium thin film evaporation process resulted in even higher value of the thin chromium film adhesion to the float glass substrate where critical normal force for coating delamination exceeded 0.2 N. Further investigation has to be carried out to determine the effect of surface preparation method and glass substrate heating parameters for adhesive bonding properties of thin chromium films.
Acknowledgments
Authors would like to thank dr. K. Šlapikas, V. Kopustinskas, dr. R. Gudaitis, dr. A. Guobienė, A. Gudonytė, dr. I. Prosyčevas (all from Institute of Materials Science of Kaunas University of Technology) and dr. G. Janušas (from International Studies Center of Kaunas University of Technology) for technical assistance. Support of State Studies Foundation is gratefully acknowledged.
REFERENCES
1. Regelskis, K., Račiukaitis, G., Gedvilas, M. Ripple Formation in the Chromium Thin Film During Laser Ablation Applied Surface Science 253 (15) 2007: pp. 6584 – 6587.
2. Lazauskas, A., Grigaliūnas, V., Meškinis, Š., Kopustinskas, V., Guobienė, A., Gudonytė, A., Andrulevičius, M., Tamulevičius, T. Hydrophobic Diamond Like Carbon Film for Surface Micromachining Materials Science (Medžiagotyra) 15 (3) 2009: pp. 196 – 200.
3. Kulkarni, A. K., Chang, L. C. Electrical and Structural Characteristics of Chromium Thin Films Deposited on Glass and Alumina Substrates Thin Solid Films 301 1997: pp. 17 – 22.
4. Jukna, T., Baltrušaitis, J., Sinkevičius, V., Viržonis, D. A Thin Chromium Film Formation Monitoring Method: Monitoring of the Early Stages Thin Solid Films 516 2008: pp. 2943 – 2947.
http://dx.doi.org/10.1016/j.tsf.2007.10.087
5. Lousa, A., Romero, E., Martinez, J., Esteve, J., Montala, F., Carreras, L. Multilayered Chromium / Chromium Nitride Coatings for Use in Pressure Die-casting Surface and Coatings Technology 146 – 147 2001: pp. 268 – 273.
6. Miller, R. A., Holland, H. J. Crystallographic Orientation of Sputtered Cr Films on Glass and Glass-ceramic Substrates Thin Solid Films 298 1997: pp. 182 – 186.
7. Mittal, K. L. Adhesion Measurement of Thin Films Electrocomponent Science and Technology 3 1976: pp. 21 – 42.
8. Ghandhi, S. K. (Ed.) VLSI Fabrication Principles: Silicon and Gallium Arsenide. 2nd ed. John Wiley, New York, 1994: 641 p.
9. Awadelkarim, O. O., Wang, Y. Z. The Impact of RCA Treatment of Glass Substrates on the Properties of Polycrystalline Silicon Thin Film Transistors Microelectronic Engineering 45 1999: pp. 299 – 310.
10. Müller-Buschbaum, P. Influence of Surface Cleaning on Dewetting of Thin Polystyrene Films The European Physical Journal E 12 2003: pp. 443 – 448.
11. Wang, Y. Z., Awadelkarim, O. O. The Effects of Glass- Substrate’s Surface-Treatment on the Characteristics of N-Channel Polycrystalline Silicon Thin Film Transistors Journal of Electronic Materials 27 (11) 1998: pp. 77 – 80. http://dx.doi.org/10.1007/s11664-998-0084-5
12. Chen, Y., Liu, W., Ye, C., Yu, L., Qi, S. Preparation and Characterization of Self-assembled Alkanephosphate Monolayers on Glass Substrate Coated with Nano-TiO2 Thin Film Materials Research Bulletin 36 2001: pp. 2605 – 2612. http://dx.doi.org/10.1016/S0025-5408(01)00731-0
13. Xianhua, C., Tao, B., Ju, W., Liang, W. Characterization and Tribological Investigation of Self-assembled Lanthanum-based Thin Films on Glass Substrates Wear 260 2006: pp. 745 – 750.
14. Eske, L. D., Galipeau, D. W. Characterization of SiO2 Surface Treatments Using AFM, Contact Angles and a Novel Dewpoint Technique Colloids and Surfaces A: Physicochemical and Engineering Aspects 154 1999: pp. 33 – 51.
http://dx.doi.org/10.1016/S0927-7757(98)00907-8
15. Guha, A., Barron, R. M., Balachandar, R. An Experimental and Numerical Study of Water Jet Cleaning Process Journal of Materials Processing Technology 211 2011: pp. 610 – 618.
16. Kolluru, P. V., Green, D. J., Pantano, C. G., Muhlstein, C. L. Effects of Surface Chemistry on Nanoindentation of Float Glass Surfaces Journal of the American Ceramic Society 93 (3) 2010: pp. 838 – 847.
17. Bartella, J., Grfinwald, H., Herwig, U. XPS and SIMS Investigations on Plasma-Treated Glass Surfaces Mikrochimica Acta 1987: pp. 365 – 369. http://dx.doi.org/10.1007/BF01199512
18. Bhattacharya, S., Datta, A., Berg, J. M., Gangopadhyay, S. Studies on Surface Wettability of Poly(Dimethyl) Siloxane (PDMS) and Glass under Oxygen-plasma Treatment and Correlation with Bond Strength Journal of Microelectromechanical Systems 14 (3) 2005: pp. 590 – 597.
19. Fridman, A. A. Plasma Chemistry. Cambridge University Press, NY, USA, 2008: pp. 531 – 548. http://dx.doi.org/10.1017/CBO9780511546075
20. Mattox, D. M. Handbook of Physical Vapor Deposition (PVD) Processing. Noyes Publications, 1998.
21. Takeda, S. Oxygen and Silver Diffusion Into Float Glass Journal of Non-Crystalline Solids 352 (36 – 37) 2006: pp. 3910 – 3913.
22. Zhang, Q. Simulation of Tin Penetration in the Float Glass Process (Float Glass Tin Penetration) Applied Thermal Engineering 2011: doi:10.1016/j.applthermaleng.2010.12. 030.
http://dx.doi.org/10.1016/j.applthermaleng.2010.12
23. Fernández Oro, J. M., Argüelles Díaz, K. M., Santolaria Morros, C., Cobo Hedilla, A. F. Multiphase Modelling of Pouring Glass Over the Spout Lip of an Industrial Float in the Flat Glass Forming Process International Journal for Numerical Methods in Fluids 58 2008: pp. 1147 – 1177.
24. Kolluru, P. V., Green, D. J., Pantano, C. G., Muhlstein, C. L. Effects of Surface Chemistry on the Nanomechanical Properties of Commercial Float Glass Journal of the American Ceramic Society Kolluru et al. 93 (3) 2010: pp. 838 – 847.
25. Goodman, O., Derby, B. The Mechanical Properties of Float Glass Surfaces Measured by Nanoindentation and Acoustic Microscopy Acta Materialia 59 (4) 2011: pp. 1790 – 1799.
26. Safonov, V., Zykova, A., Smolik, J., Rogovska, R., Donkov, N., Georgieva, V. The Surface Parameters Modifications at Nano Scale for Biomedical Applications Journal of Physics: Conference Series 253 2010:
doi:10.1088/1742-6596/253/1/012068.
27. Chi, F. T., Jiang, K., Li, B., Jiang, B. Properties of Electroless Ni-P Deposition of Glass Substrates Using a Mercapto Organosilanes for Functional Groups to Graft Palladium Applied Surface Science 255 (5) 2008: pp. 2740 – 2745.
28. Cras, J. J., Rowe-Taitt, C. A., Nivens, D. A., Ligler, F. S. Comparison of Chemical Cleaning Methods of Glass in Preparation for Silanization Biosensors and Bioelectronics 14 (8 – 9) 1999: pp. 683 – 688.
29. Choi, S-W., Choi, W-B., Lee, Y-H., Ju, B-K. Effect of Oxygen Plasma Treatment on Anodic Bonding Journal of the Korean Physical Society 38 (3) 2001: pp. 207 – 209.
30. Lim, K-B., Lee, D-C. Surface Modification of Glass and Glass Fibres by Plasma Surface Treatment Surface and Interface Analysis 36 2004: pp. 254 – 258.
31. Herminghaus, S. Wetting: Introductory Note Journal of Physics: Condensed Matter 17 (9) 2005: pp. 261 – 264. http://dx.doi.org/10.1088/0953-8984/17/9/E01
32. Young, T. An Essay on the Cohesion of Fluids Philosophical Transactions. Royal Society Publishing 95 1905: pp. 65 – 87.
33. Stalder, A. F., Kulik, G., Sage, D., Barbieri, L., Hoffmann, P. A Snake-based Approach to Accurate Determination of Both Contact Points and Contact Angles Colloids and Surfaces A: Physicochemical and Engineering Aspects 286 (1 – 3) 2006: pp. 92 – 103.
34. Stalder, A. F. DropSnake, Biomedical Imaging Group, EPFL, [ON LINE] visited 2011: http://bigwww.epfl.ch/demo/dropanalysis.
35. ImageJ, [ON LINE] visited 2011. http://rsb.info.nih.gov/ij/.
36. Perry, A. J. The Adhesion of Chemically Vapour-deposited Hard Coatings to Steel – the Scratch Test Thin Solid Films 78 (1) 1981: pp. 77 – 94.
37. Hintermann, H. E. Adhesion, Friction and Wear of Thin Hard Coatings Wear 100 (1 – 3) 1984: pp. 381 – 397.
38. Bull, S. J., Rickerby, D. S. New Developments in the Modelling of the Hardness and Scratch Adhesion of Thin Films Surface and Coatings Technology 42 (2) 1990: pp. 149 – 164.
39. Bull, S. J., Berasetegui, E. G. An Overview of the Potential of Quantitative Coating Adhesion Measurement by Scratch Testing Tribology International 39 (2) 2006: pp. 99 – 114.
40. Buijnsters, J. G., Shankar, P., van Enckevort, W. J. P., Schermer, J. J., ter Meulen, J. J. Adhesion Analysis of Polycrystalline Diamond Films on Molybdenum by Means of Scratch, Indentation and Sand Abrasion Testing Thin Solid Films 474 2005: pp. 186 – 196.
http://dx.doi.org/10.1016/j.tsf.2004.09.021
41. Ponelytė, S., Prosyčevas, I., Guobienė, A., Balčiūnas, R., Puišo, J. Formation of MEMS Nanocomposit Layers and Investigation of Their Mechanical Properties Mechanics (Mechanika) 2 (76) 2009: pp. 77 – 82.
42. Takeda, S., Yamamoto, K., Hayasaka, Y., Matsumoto, K. Surface OH Group Governing Wettability of Commercial Glasses Journal of Non-Crystalline Solids 249 1999: pp. 41 – 46.